Tandem Mobi System
Move in ways you never thought possible
LITTLE PUMP, LOTS OF CONTROL
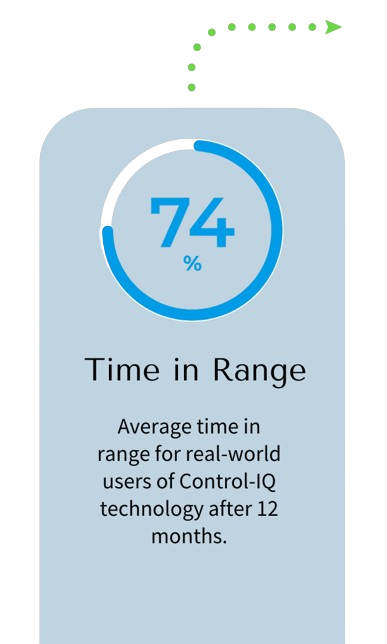
Predicts and Helps Prevent Highs & Lows
Tandem Mobi is powered by Control‑IQ technology, which can help keep blood sugars in range by using CGM* values to predict glucose levels 30 minutes ahead and automatically adjust insulin delivery, including automatic correction boluses.†
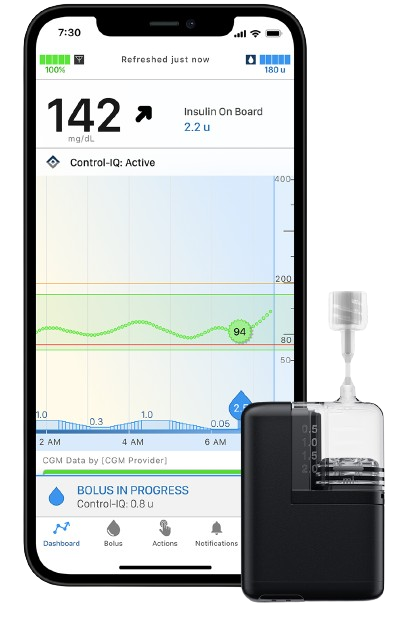
iPhone Control
Tandem Mobi is the only automated insulin delivery system fully controllable via a mobile app.‡

Wearability Like Never Before
Choose How to Wear Tandem Mobi
The pump is so small that it can be worn almost anywhere, giving you greater discretion, comfort, and options for how you manage your diabetes.

Clip it
Clip it easily to your clothes, dress, workout gear, or pajamas. Tandem Mobi can be worn almost anywhere.
Slip it
The impressively small Tandem Mobi is so discreet that you can simply slip it into the coin pocket of your jeans (yes, it's that small).
Wear it
Wear it on-body with an adhesive sleeve (sold separately), which is compatible with all infusion sets, including the AutoSoft XC with 5-inch tubing.
Disconnect. Reconnect.
It's a snap!
It's easy to temporarily disconnect an infusion set from your Tandem Mobi for showering, hot tubs, or contact sports.
Life is Better with Options
CGM Sensors
The Tandem Mobi system currently integrates with Dexcom G6, and integration with Dexcom G7 is planned for late spring of 2024.^
Infusion Sets
The system is compatible with all Tandem infusion sets, so you can choose the length, angle, and material that best fits your lifestyle.
Order Tandem Mobi Today!
Remote Software Updates
Diabetes technology evolves at a rapid pace. Tandem insulin pumps are capable of remote feature updates allowing you to keep your pump up-to-date with the latest technology.††
Reporting Made Simple
The Tandem Source platform was created to give you access to all things Tandem Diabetes Care in one convenient platform. See all of your therapy data and easily reorder supplies for your pump.
Which Tandem insulin pump is right for you?
Tandem Diabetes Care is the #1 recommended insulin pump brand by people living with diabetes — four years and counting!2 Choose which Tandem automated insulin delivery system best fits your lifestyle.

Responsible Use of Control‑IQ Technology
Even with advanced systems such as Control-IQ technology, you are still responsible for actively managing your diabetes. Control-IQ technology does not prevent all high and low blood glucose events. The system is designed to help reduce glucose variability, but it requires your accurate input of information, such as meals and periods of sleep or exercise. Control-IQ technology will not function as intended unless you use all system components, including your CGM, infusion sets and pump cartridges, as instructed. Importantly, the system cannot adjust your insulin dosing if the pump is not receiving CGM readings. Because there are situations and emergencies that the system may not be capable of identifying or addressing, always pay attention to your symptoms and treat according to your healthcare provider’s recommendations.
* CGM sold separately.
† If glucose values are predicted to be above 180 mg/dL, Control-IQ technology calculates a correction bolus using the Personal Profile settings and a target of 110 mg/dL and delivers 60% of that value.
‡ Our mobile apps require a compatible smartphone model and operating system (sold separately). Only available to pump users who reside in the U.S.
§ Do not wear or place your pump more than 12 inches (30.5 cm) above your infusion site. Doing so may result in over delivery of insulin.
^ Tandem Mobi integration with Dexcom G7 is under development. The safety and effectiveness of Dexcom G7 with Tandem Mobi has not yet been established. Tandem Mobi is intended for use with Dexcom G6 CGM.
** Tested at 8 feet for 2 hours (IP28 rating).
†† Future updates for all or some Tandem products may not be developed and may not be offered everywhere and would be subject to applicable regulatory approvals. Software updates are only available to customers who are in warranty at the time they update their pump. Additional training may be required to access certain software updates. Charges may apply. Tandem may discontinue select software and features over time at its discretion.
References
1. Breton MD, Kovatchev BP. One year real-world use of the Control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther. 2021;23(9):601-608. doi: 10.1089/dia.2021.0097.
2. dQ&A US Diabetes Connections Patient Panel Report; Net Promoter Score; Jan. 2019-Sept. 2023: P.49, Sept. 2023.
Important Safety Information
RX ONLY.
Indications for Use
Tandem Mobi system: The Tandem Mobi insulin pump with interoperable technology (the pump) is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in persons requiring insulin. The pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The pump is intended for single patient, home use and requires a prescription. The pump is indicated for use in individuals 6 years of age and greater.
t:slim X2 insulin pump: The t:slim X2 insulin pump with interoperable technology is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in people requiring insulin. The pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The pump is intended for single patient use. The pump is indicated for use with NovoLog or Humalog U-100 insulin. The pump is indicated for use in individuals 6 years of age and greater.
Control-IQ technology: Control-IQ technology is intended for use with compatible integrated continuous glucose monitors (iCGM, sold separately) and alternate controller enabled (ACE) pumps to automatically increase, decrease, and suspend delivery of basal insulin based on iCGM readings and predicted glucose values. It can also deliver correction boluses when the glucose value is predicted to exceed a predefined threshold. Control-IQ technology is intended for the management of Type 1 diabetes mellitus in persons 6 years of age and greater. Control-IQ technology is intended for single patient use. Control-IQ technology is indicated for use with NovoLog or Humalog U-100 insulin.
Warning: Control-IQ technology should not be used by anyone under the age of 6 years old. It should also not be used in patients who require less than 10 units of insulin per day or who weigh less than 55 pounds.
Control-IQ technology is not indicated for use in pregnant women, people on dialysis, or critically ill patients. Do not use Control-IQ technology if using hydroxyurea. Users of a Tandem insulin pump and Control-IQ technology must use the insulin pump, CGM, and all other system components in accordance with their respective instructions for use; test blood glucose levels as recommended by their healthcare provider; demonstrate adequate carb-counting skills; maintain sufficient diabetes self-care skills; see healthcare provider(s) regularly; and have adequate vision and/or hearing to recognize all functions of the pump, including alerts, alarms, and reminders. The Tandem pump must be removed before MRI, CT, or diathermy treatment. Visit tandemdiabetes.com/safetyinfo for additional important safety information.
The Tandem Source platform is intended for use by individuals with diabetes mellitus who use Tandem Diabetes Care insulin pumps, their caregivers, and their healthcare providers in home and clinical settings. The Tandem Source platform supports diabetes management through the display and analysis of information uploaded from Tandem insulin pumps.